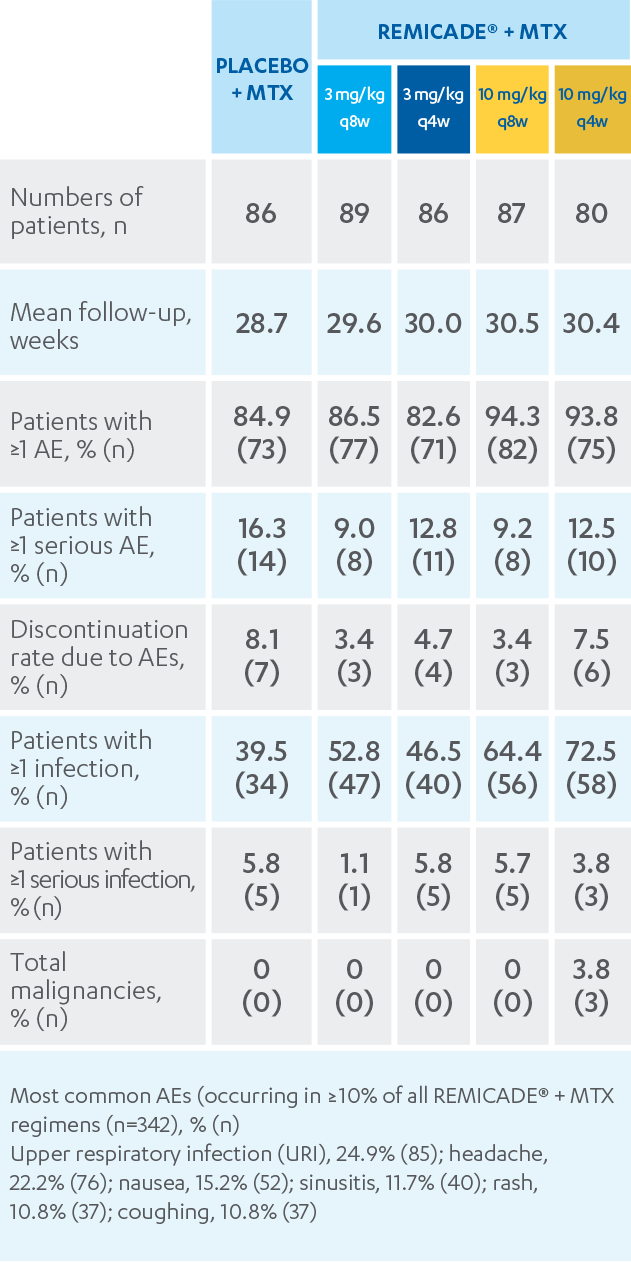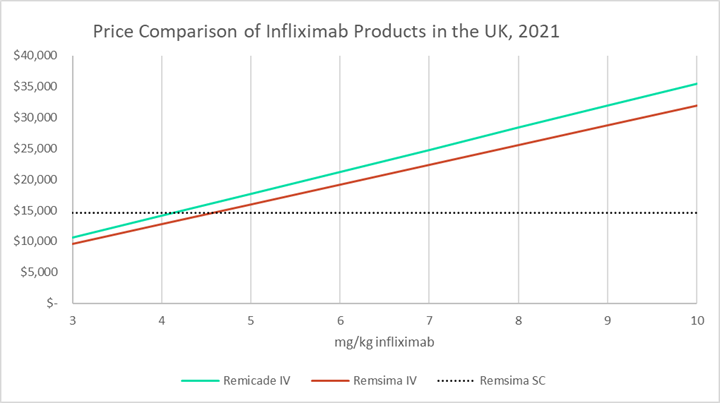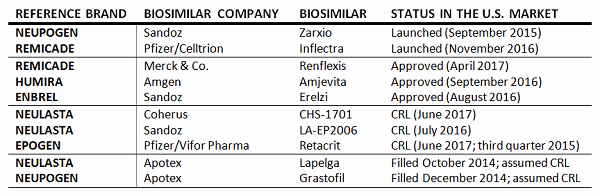
Report on EU's Experience with Biosimilar Drugs Released: Will U.S. Experience Be Similar? - Page 2 of 5 - The Rheumatologist

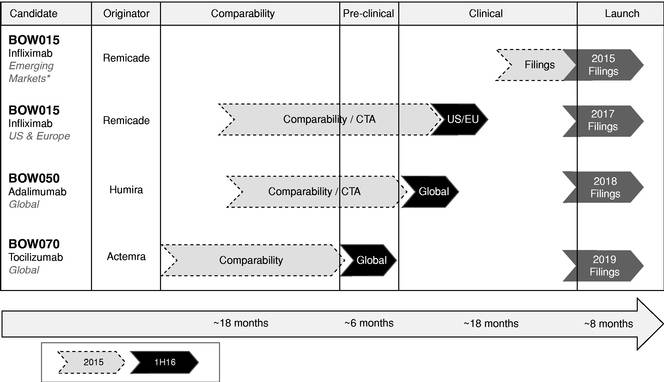
Biosimilar strategy for infliximab by MedDrive™ includes the reference drug - Prime Therapeutics LLC

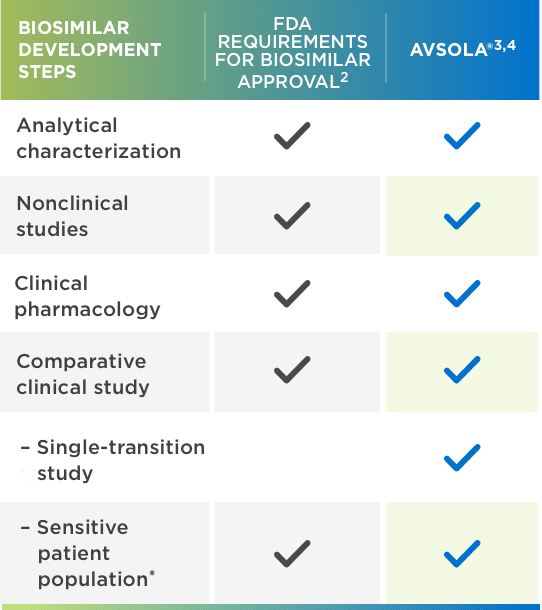
U.S. Biosimilar Launches Accelerate with Five Launches in Q4 2019 and early 2020 | Biosimilars Law Bulletin