The EffecTs of Amlodipine and other Blood PREssure Lowering Agents on Microvascular FuncTion in Small Vessel Diseases (TREAT-SVDs) trial: Study protocol for a randomised crossover trial - Anna Kopczak, Michael S Stringer,

Methodology of a large prospective, randomised, open, blinded endpoint streamlined safety study of celecoxib versus traditional non-steroidal anti-inflammatory drugs in patients with osteoarthritis or rheumatoid arthritis: protocol of the standard care ...
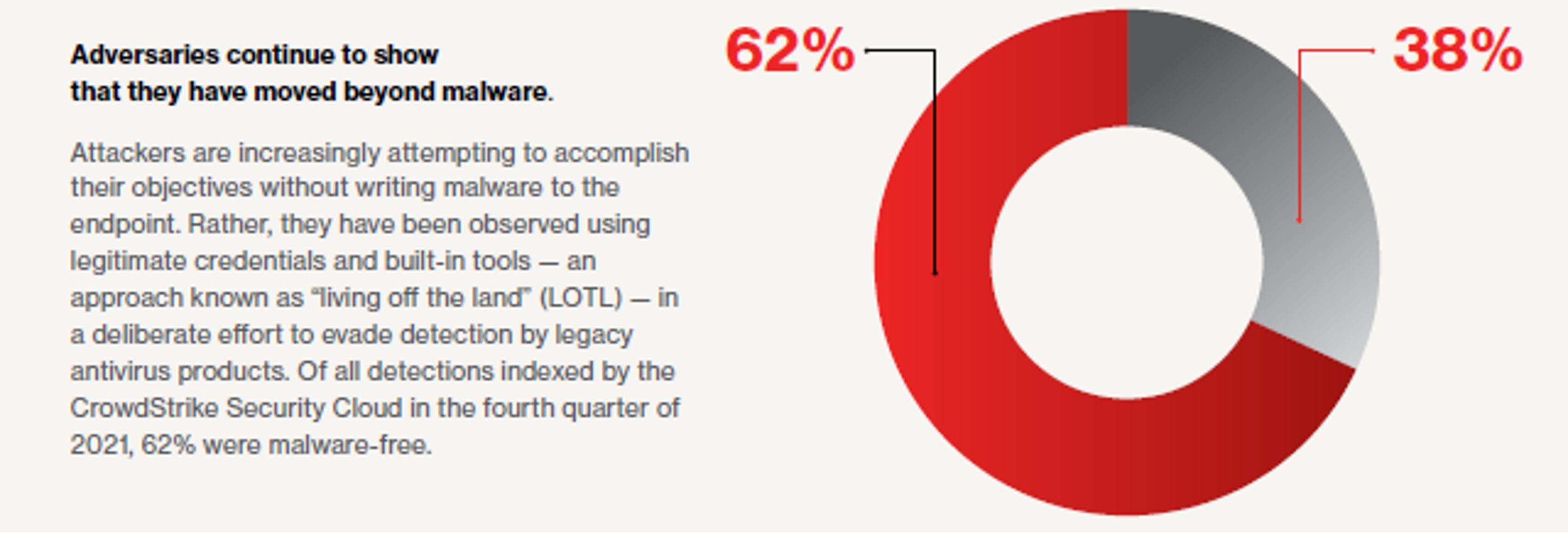
![PDF] Subjective endpoints in clinical trials: the case for blinded independent central review | Semantic Scholar PDF] Subjective endpoints in clinical trials: the case for blinded independent central review | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/9b64e5348e0e0732cebba88e8435a8b2465f45f9/2-Table1-1.png)
PDF] Subjective endpoints in clinical trials: the case for blinded independent central review | Semantic Scholar

Protocol of the Febuxostat versus Allopurinol Streamlined Trial (FAST): a large prospective, randomised, open, blinded endpoint study comparing the cardiovascular safety of allopurinol and febuxostat in the management of symptomatic hyperuricaemia –

Intensive blood pressure control after endovascular thrombectomy for acute ischaemic stroke (ENCHANTED2/MT): a multicentre, open-label, blinded- endpoint, randomised controlled trial - The Lancet

A randomized, multicenter, open-label, blinded end point trial comparing the effects of spironolactone to chlorthalidone on left ventricular mass in patients with early-stage chronic kidney disease: Rationale and design of the SPIRO-CKD

Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial - The Lancet Neurology

Heart Rehabilitation in patients awaiting Open heart surgery targeting to prevent Complications and to improve Quality of life (Heart-ROCQ): study protocol for a prospective, randomised, open, blinded endpoint (PROBE) trial | BMJ

Study overview. *PROBE: prospective randomized open blinded endpoint... | Download Scientific Diagram

Endovascular treatment versus no endovascular treatment after 6–24 h in patients with ischaemic stroke and collateral flow on CT angiography (MR CLEAN-LATE) in the Netherlands: a multicentre, open-label, blinded-endpoint, randomised, controlled, phase

Design of Major Randomized Trials: Part 3 of a 4-Part Series on Statistics for Clinical Trials - ScienceDirect